 |
| Today's Chemistry Cat! |
- all chemical reactions involve a change in energy
- some absorb:
indothermicendothermic - some release:
exitthermicexothermic - molecules are held together by chemical bonds
- add energy to break bonds
- give off energy to make bonds
reaction takes more energy to break then to make (bonds)= exothermic
reactions takes less to break then make (bonds) =
endothermic
Endothermic and Exothermic Reactions
Entropy: heat contained in a system
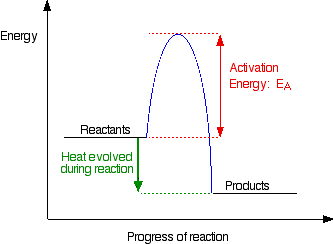
Energy Diagrams
- we can graph potential energy of chemicals as they under go a reaction
- relative amounts of energy determine if a reaction is endothermic or exothermic
Activation Energy: energy that must be added to make the reaction to occur
DeltaH (change in entropy): change in potential energy during reaction (products - reactants)
Endothermic Energy Diagrams
- products will have more energy then the reactants (deltaH will be positive)
Exothermic Energy Diagrams
- products will have less energy then the reactants (deltaH will be negative
Try these!
exothermic vs endothermic quiz
this has the same ideas as above
The first 6 questions are just looking at the graph and saying the answer.
The last 6 questions are as if the graph is flipped...the graph now starts at what you before considered the end and goes TOWARD the y-axis. Good luck :)

No comments:
Post a Comment