Since the 5th century, a few elements had been discovered, including gold, tin, silver, lead and mercury, as these were easy to find. The first discovery was by Henning Brand, a German man, on a wild chase for the philosophers stone. In 1669, he discovered phosphorus, but kept it a secret, and the rock was later publicized in 1680 by Robert Boyle.
A hundred years later, a chemist named Antoine Lavoisier lived. He created a text book of elements that could not be broken down, which also included light, and caloric, which were then believed to be material substances.
Lavoisier's elements
Gases
|
New names (French)
|
Old names (English translation)
|
Lumière
|
Light
|
Calorique
|
Heat
Principle of heat
Igneous fluid
Fire
Matter of fire and of heat
|
Oxygène
|
Dephlogisticated air
Empyreal air
Vital air
Base of vital air
|
Azote
|
Phlogisticated gas
Mephitis
Base of mephitis
|
Hydrogène
|
Inflammable air or gas
Base of inflammable air
|
|
Metals
|
New names (French)
|
Old names (English translation)
|
Antimoine
|
Antimony
|
Argent
|
Silver
|
Arsenic
|
Arsenic
|
Bismuth
|
Bismuth
|
Cobolt
|
Cobalt
|
Cuivre
|
Copper
|
Étain
|
Tin
|
Fer
|
Iron
|
Manganèse
|
Manganese
|
Mercure
|
Mercury
|
Molybdène
|
Molybdena
|
Nickel
|
Nickel
|
Or
|
Gold
|
Platine
|
Platina
|
Plomb
|
Lead
|
Tungstène
|
Tungsten
|
Zinc
|
Zinc
|
|
Nonmetals
|
New names (French)
|
Old names (English translation)
|
Soufre
|
Sulphur
|
Phosphore
|
Phosphorus
|
Carbone
|
Pure charcoal
|
Radical muriatique
|
Unknown
|
Radical fluorique
|
Unknown
|
Radical boracique
|
Unknown
|
|
Earths
|
New names (French)
|
Old names (English translation)
|
Chaux
|
Chalk, calcareous earth
|
Magnésie
|
Magnesia, base of Epsom salt
|
Baryte
|
Barote, or heavy earth
|
Alumine
|
Clay, earth of alum, base of alum
|
Silice
|
Siliceous earth, vitrifiable earth
|
|
By 1809, 47 elements had been discovered. Chemists noted pattern in reactions, and attempted to classify them.
In 1862, the first form of element organization is published by a French chemist, Alexandre-Émile Béguyer de Chancourtois, that appeared to be a spiral, with increasing mass downward.
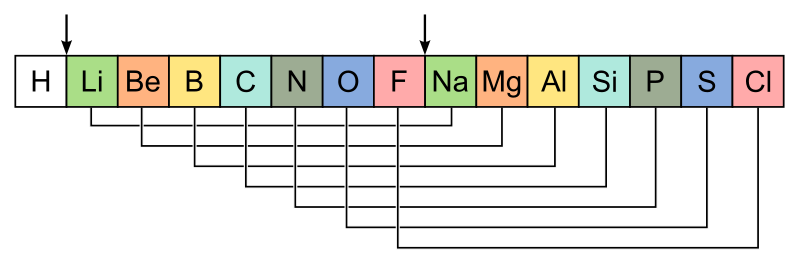
1864 John Newland proposes the Law of Octaves, which he found by assigning a mass of 1 to helium, and then ordering the rest by mass. This lead to the discovery that every 8th element had similar properties,
in 1869, Mendeleev published the first official periodic table. He ignored certain previous ideas, and instead grouped certain elements together according to properties, vs the previous method of weight organization. Mendeleev predicted atomic weights of yet to be discovered elements, and left empty sections in his table to accommodate them
In 1911, Ernest Rutherford publishes a paper that explained nuclear charges. Later that year, Antonius Van Den Broek publishes a paper relating the atomic weight of an element to the charge of the atom. This became the basis of organization of the table, the atomic number.
Two years later, Henry Moseley proposes that the wavelengths of x-ray emissions of elements is directly proportional to that of the atomic number.
The latest changes made were by Glen Seaborg, who discovered plutonium, and elements 94-102. It was his doing that resulted in the lanthanide and actanide series' being placed below the table.

 (if you get a negative answer, then think of what you have as having absolute value bars around it, just make it positive)
(if you get a negative answer, then think of what you have as having absolute value bars around it, just make it positive)