Generally make up the most reactive part of the molecule
Can be either single bonds or group bonds
Single added atoms are generally called HALOGENATED COMPOUNDS, while grouped atoms are NITRO COMPOUNDS.
HALOGENATED:
F: fluoro
Cl: chloro
Br: bromo
I: iodo
General trend:
-Do not dissolve in water
-Hydrocarbons with fluorine are I reactive
-Hydrocarbons with chlorine and bromine are reactive, but only in drastic conditions
-Hydrocarbons with iodine are very reactive
 |
| 1,3-dibromo-1,1-difluoropropane |
NITRO:
NO2: nitro
General trend:
-Usually insoluble in water
-Generally do not react
-Explosive
-Usually have a pleasant odor
**naming**
Follow the same rules as simple hydrocarbons. Can be combined with alkanes, alkenes and alkynes. If there are multiple groups, precede with the prefixes mono-, di-, tri-, tetra-...
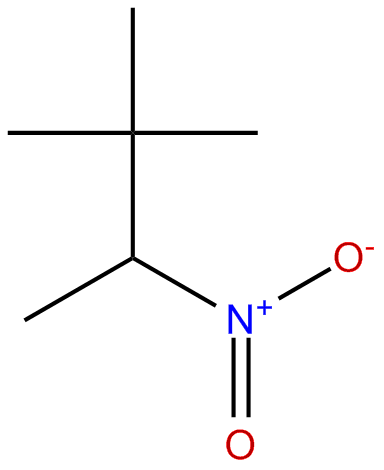 |
| 2,2-dimethyl-3-nitrobutane |
ALCOHOL:
Organic compounds containing hydroxide (OH)
General trend:
-Poisonous to some degree
-While OH is soluble in water, the hydrocarbon chain is not. The longer the chain, the less soluble in water it is.
**naming**
Replace the ending "e" with the suffix "-ol"
If there are multiple, add the previous mentioned prefixes before the "ol" (1 hydroxide bond: propanol. 2 hydroxide bonds: 2,3-propanediol • the "e" remains if there are multiple • identify the placement of the OH with preceding numbers.
| Hexanol |
ALDEHYDES:
Contains carbonyl (a double group)
Has a double bonded oxygen at the end of the chain
General trend:
-Partially soluble in water
-Very reactive
**naming**
Replace "e" at end with -al suffix
Simplest form: methanal
 |
| Ethanal |
KETONES:
Double bonded carbon not at end of the chain
General trend:
-Partially soluble in water
-Unreactive
**naming**
Replace e ending with -one suffix
 |
| Propanone |
CARBOXYLIC ACIDS:
Has a double bonded oxygen and hydroxide at end of chain.
General trend:
-Can be neutralized when placed with an acid.
**naming**
Drop the "e" and replace with -oic acid
Simplest form: methanoic acid
| 4-bromo-2fluorobutanoic acid |
ESTERS:
oxygen bond dividing an acyl from an alkyl. Has a double bonded oxygen attached to closest carbon to oxygen
General trend:
-Pleasant smell
-Can be converted back into alcohol and carboxylic acid
**naming**
Alkyl: name using same rules
Acyl: (the remainder) replace -oic acid suffix of the corresponding carboxylic acid with -oate
| Propyl Heptanoate |
Oxygen group connecting two alkyl groups.
General trend:
-Highly flammable
-Insoluble in water
-Makes a good solvent for organize compounds
-Certain compounds (ethoxy ethane) have anesthetic properties
**naming**
O is where side group counting begins
Follow all basic rules, but replace -yl with -oxy to the side group names
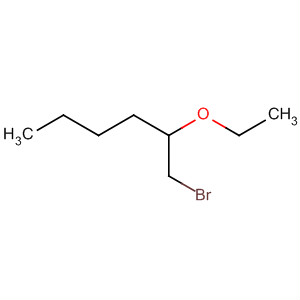 |
| 1-bromo-2-ethoxy hexane |
AMINES:
Contains nitrogen compounds bonded to either hydrogen or carbon
General trend:
-Organic bases ➡ form salts easily when reacted with water
-Closely related to NH3
-Fishy odor
**naming**
Follow standard rules, but the longest chain name is preceded by amino.
As always, prefix numbers are used to identify the location of the amine.
ALCYLICS
Can form cycloalkanes, cycloalkenes and cycloalkynes. Forms a ring with carbons, either a branch or a main chain. Will also support other branches
General trend:
-Less stable (more reactive)
-can be branched
**naming**
numbering can begin anywhere, and go any direction
lowest chains possible
1. count total carbons, and name accordingly. Add cyclo prefix
2. if there is only one side group, it is assumed to be one, and no number is needed
3. If there are multiple, name the first group with number 1
4. side groups are named following basic rules
5. for alkenes, alkynes the bond is assumed to be the first, and no number is needed unless there are multiple
6. if the side groups are tied, give the lowest number to the first alphabetically ordered group
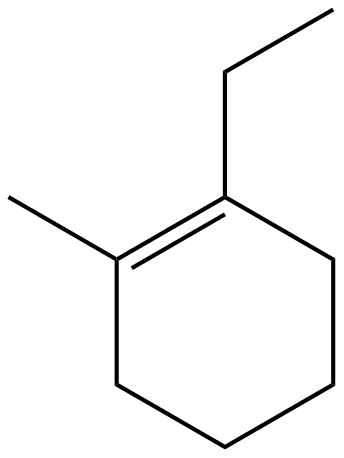 |
| 1-ethyl-2-methylcyclohexene |
AROMATICS
contains a benzene ring
General Trend:
-Electrons are "delocalized" meaning that they can change position through the ring
-All of the carbon bonds therefore have the same reactivity
-Less reactive that cycloalkenes/ynes
-Because of the delocalization, benzene is very stable
Has a special diagram because of the free movement of electrons
 |
| Benzene |
**naming**
When it is the main chain, just name the side groups attached to it, followed by benzene
If it makes up a side group, then it is called phenyl.
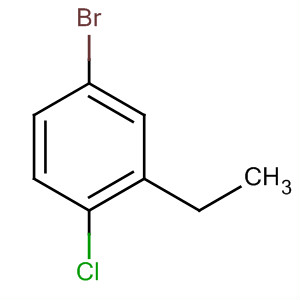 |
| 4-bromo-1-chloro2-ethyl benzene |
No comments:
Post a Comment